Sairopa Presents Promising ADU-1604 Clinical Data at ESMO Congress 2025: Novel CTLA-4 Blocking Antibody Demonstrates Benchmark Efficacy with Improved Safety Profile in Melanoma Patients
October 20, 2025 – ROTTERDAM, Netherlands – Sairopa, a...

Sairopa is a clinical-stage company that develops novel treatments for cancer by modulating the patient’s immune system. Cancer treatment was revolutionized by the introduction of so-called immune checkpoint inhibitors, that enhances the immune response against the patient’s own tumor. However only about 30% of cancer patients find benefit from such therapies. It is well accepted that for a productive anti-cancer immune response to happen, all elements of the cancer-immunity cycle need to be functional: tumor-antigen release, antigen-processing, -presentation, T-cell activation and blockade of local immunosuppression in the tumor.
Early 2021 Sairopa acquired, backed by Van Herk Investments, a portfolio of therapeutic antibodies from Chinook Therapeutics. These antibodies were originally developed by BioNovion/Aduro Biotech Europe using the proprietary B-Select antibody platform that was also used to identify pembrolizumab (Keytruda™), BION-1301 and MK-5890 (Guelen et al., 2022).
It is Sairopa’s mission to develop agents that can modulate this cancer-immunity cycle on different points:
ADU-1805, Sairopa’s proprietary anti-SIRPalpha antibody is enhancing tumor cell uptake and presentation to the T cells to induce priming and activation (Voets et al., 2019).
ADU-1604, Sairopa’s proprietary and unique anti-CTLA4 antibody is enhancing T cell priming and activation to raise a higher diversity of tumor cytotoxic immune cells. ADU-1604 is currently being tested in Phase 1 Clinical trials to assess for safety and efficacy in patients suffering from advanced melanoma.
Discovery Programs (undisclosed) that inhibit local immunosuppression in the tumor.
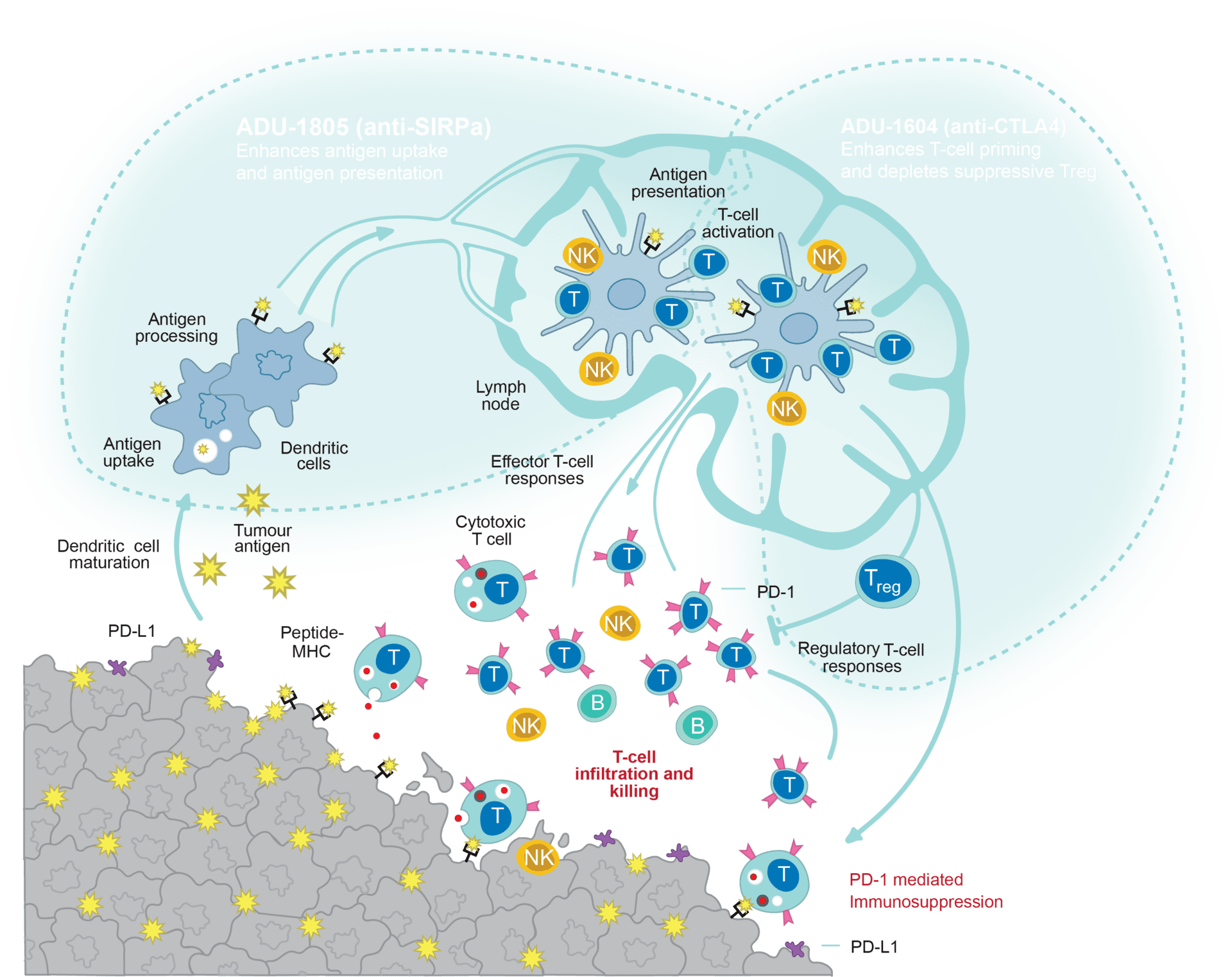
Sairopa programs target distinct parts of the cancer-immunity cycle (adapted from Chen and Mellman, 2013)
Sairopa’s team has a leading reputation in the field of immune oncology and is laser-focused to deliver the therapeutic antibodies towards clinical benefit in cancer patients.
The company is based in Rotterdam, the Netherlands.

Sairopa’s team has a leading reputation in the field of immune oncology and is laser-focused to deliver the therapeutic antibodies towards clinical benefit in cancer patients.
The company is based in Rotterdam, The Netherlands.

Managing Director
Dharminder is Managing Director of Sairopa, he was involved in the foundation and financing of Sairopa. Furthermore, Dharminder is owner and managing director of Exponential B.V. in which capacity he acts as consultant to Van Herk Investments and companies in the Van Herk Investments portfolio. Furthermore, he is CEO and co-founder of SkylineDx a company based in Rotterdam, The Netherlands, and developing diagnostic tests in oncology. Mr. Chahal has built an extensive network in the global life science industry and created a strong track record in supporting successful companies in their journeys. Currently the main investments in the portfolio are Galapagos, Zealand Pharma (Denmark) and BioInvent (Sweden).
As a board member or advisor he has been and still is active for several European companies and funds in the field of life sciences. Mr. Chahal previously held various positions in investment banking and asset management, including Kempen & Co and Robeco. Mr. Chahal obtained a master degree in Business Economics (Erasmus University Rotterdam) and a master degree cum laude in Aerospace Engineering (TU Delft).

Chief Business Officer
Gurvinder is owner and director of Vinco Vita BV. Mr Chahal started his career as a management trainee with the ING Group and has spent 18 years in corporate banking, in several relationship management roles, both in the Netherlands and India for large multinational banks. Mr. Chahal is the Chief Business Officer and Chief Financial Officer of Sairopa BV since foundation of Sairopa BV. Mr. Chahal obtained a master degree in Business Economics (Erasmus University Rotterdam).

Executive Assistant & Office Manager
Deborah has over a decade of experience in the biotech industry supporting teams and management in the day to day business (SkylineDx). Deborah has a strong eye for team dynamics and a pragmatic problem solving mindset. With her Human Resources knowledge and hands on mentality she is invaluable for a strong backbone of the company. She also works as a Personal Assistant to the Chief Executive Officer, acting as a first point of contact for callers, controlling access, managing diaries, the bridge between work floor and management and support with daily time management. In addition she provides assistance for board member meetings and business development

Jessica Kundapur is an experienced clinical trial manager with expertise in global Phase I-III clinical studies. After completing her Master’s degree in Biology, she coordinated investigator-initiated late-stage oncology studies at the Canadian Cancer Trials Group in Kingston, Canada. Later she joined PRC Clinical, a CRO in San Francisco, USA where she specialized in site management for several trials in different therapeutic areas. In the Netherlands, she first worked for Seuss Consulting and gained business development, and marketing experience as a consultant for pharmaceutical and CRO companies. Combining this unique perspective along with her clinical operations experience, she joined biotechnology companies (Rainier Therapeutics, Byondis B.V.) and successfully managed Early and Late Phase Oncology studies. Leveraging her international background and diverse experiences, Jessica excels in cultivating strong stakeholder relationships to smoothly execute clinical trials and to achieve strategic objectives.

Chief Operating Officer
Laura is an experienced clinical professional in Early Oncology and Late Stage Oncology, who started her career in 2006 at an international CRO and has worked at the development of several successful assets in the past 16 years. Due to her experience at various international Pharmaceutical companies (MSD, Acerta Pharma, Astra Zeneca) and Clinical Contract Research Organizations (PPD, ICON) and having worked with and for several partners (Medarex-BMS, IQVIA, Amgen, Pfizer, Lilly Oncology) she is an expert in the strategy and development of clinical trials. With a Master in Health Sciences, specialized in business and health law she has an unique vision on the landscape. Laura is a builder of high performing teams, being a strong advocate for teamwork across companies and international frontiers.

Preclinical and Pharmacology consultant
Joost Kreijtz is Chief Development Officer at Trained Therapeutix Discovery, a preclinical stage biotech company. Prior to that he was Chief of Staff at Aduro Biotech Europe, working with project teams in the Netherlands and US to drive Aduro’s therapeutic antibody programs forward. During his time at Aduro, Joost was responsible for the preclinical development of ADU-1604 (co-inventor), late-stage preclinical development of ADU-1805 and the preclinical oversight for the BION-1301 program (Chinook Therapeutics). Joost started his career at the Viroscience Lab at the Erasmus Medical Center Rotterdam where he completed a PhD in immunovirology and brought a viral vector-based vaccine against highly pathogenic avian influenza from bench to bedside in the form of a Phase I/IIa clinical trial.

As Alliance Manager for Sairopa, Anton applies his passion for innovation in healthcare and his pragmatic approach to support the company’s strategic partnerships and collaborations, in order to bring them to their full potential. Thanks to his extensive experience leading multidisciplinary early- and commercial- stage projects in the pharmaceutical and biotech industries, as well as public-private partnerships, Anton is able to drive results and navigate complex relationships focusing on progress, while keeping all parties aligned and building productive relationships based on trust.

Maaike is a clinical development professional with over 20 years of experience in the pharmaceutical and biotech industry. She has a strong track record of leading global teams to successfully execute clinical trials and programs in oncology and many other therapeutic areas. Before joining Sairopa, Maaike worked in lead operational, scientific, and strategic roles for other biotechnology companies such as Byondis, Aduro Biotech and UniQure. Prior to that she worked at pharmaceutical companies including Merck and Organon in various global clinical trial management positions. Maaike holds a MSc in Clinical Research from Cardiff University in Wales and a BSc in Biochemistry.

Chief Scientific Officer
After his tenure at Organon, Schering-Plough and MSD, Hans co-founded BioNovion that was acquired by Aduro Inc. in 2015. Until 2019 he served as executive vice-president antibody research and site-head of Aduro Biotech Europe. Later that year he joined AIMM Therapeutics as chief scientific officer and is currently independent consultant in biotechnology. Hans has a strong track record in developing novel therapies in immunology and immune oncology and is (co-)inventor of pembrolizumab (Keytruda), MK-5890 (anti-CD27), BION-1301 (anti-APRIL), ADU-1604 (anti-CTLA4) and ADU-1805 (anti-SIRPalpha). Hans van Eenennaam serves on the Board of Directors of Lygature, Catalym GmBH and ISD Immunotech AsP.

Prof. Eggermont is appointed Chief Scientific Officer of the Princes Máxima Center for pediatric oncology in the Netherlands and serves on the Board of the Comprehensive Cancer Center München, Germany. Prior he was General Director of Gustave Roussy Cancer Campus Grand Paris. Here, he developed Europe’s largest phase 1-2 drug and immunotherapy development program. Prof. Eggermont is Professor Clinical & Translational Immunotherapy, UMC Utrecht, Utrecht University, NL and previously was a Professor of Oncology (Paris-Sud University) and Professor of Oncological Surgery (Erasmus University Rotterdam).

Bioanalytical and safety advisor
Julien Villaudy is co-founder and co-owner of J&S Preclinical Solutions, a consultancy firm specialized in supporting small- to mid-sized companies in their non-clinical package for IND/CTA and NDA/BLA. Julien is a pharmacology and bioanalytic expert with over 14 years of experience in academia and biotechs. Prior he was the head of pharmacology at AIMM Therapeutics were he lead the preclinical activities of their lead compound.

Senior Toxicological Consultant
Sjeng Horbach is co-founder and co-owner of J&S Preclinical Solutions, a consultancy firm specialized in supporting small- to mid-sized companies in their non-clinical package for IND/CTA and NDA/BLA. He is a Toxicology expert with over 35 years of experience. Before starting offering consultancy service, he was employed by CROs, universities and pharmaceutical companies. Most recently, he was the head of the Non-clinical Safety and Toxicology group at Genmab in The Netherlands. Sjeng has a broad experience in preclinical development of pharmaceuticals, both small molecules, biologicals and gene therapy. The projects he has been involved in cover a wide spectrum of therapeutic areas, such as CNS, oncology and fertility.
Chief Production Officer

Teun has over 25 years of CMC experience in biotechnology industry from pre-clinical to Phase 3, and commercialization. He was previously employed as Vice President Manufacturing and Supply Chain at AM-Pharma with its Phase 3 ilofotase alfa program. Prior to that he was Vice President CMC Technical Operations at Aduro Biotech Inc with responsibilities in the US and EU. From 2006 to 2016 he was with Merck (MSD) in Late Stage Process Development and Commercialization and his early career was with Unilever and BAC, in Microbial Process Development. Teun holds a Master’s degree in Bioprocess Technology from Wageningen University and completed a PhD in Cell Biology at the University of Utrecht.

Project Manager
As owner of SLC Consultancy and with over 14 years of experience in both clinical and cross-functional project management in biotechnology companies (Amgen, BioMarin, Aduro Biotech Europe, Rainier Therapeutics), Simone is overseeing the cross-functional activities of our clinical programs and supporting the clinical team in the execution of the ongoing clinical trials.

Director CMC
Maarten Friesen is Director CMC at Sairopa B.V. with over 25 years of experience in biologics drug development and manufacturing, with extensive knowledge on the early and late-stage development and commercialization of biologics. As a CMC professional, Maarten ensures the development and manufacturing of high-quality products, adhering to strict regulatory standards and timelines, to deliver safe and effective treatments to patients.
Prior to Sairopa B.V., he was Director Quality Control & Analytical Development at AM-Pharma B.V., a Dutch Phase 3 biologics pharmaceutical company. From 2017 to 2020 he was Senior Manager Quality Control at Aduro Biotech Inc. with responsibilities in the US and EU. He was with Organon, Schering-Plough and MSD (Merck) from 2001 to 2016 at numerous positions in Bioanalytical Development and Biotech Late Stage Development.

Clinical Study Manager
Jenneke brings over 15-years of both pre-clinical and clinical research experience, particularly in immune-oncology and is passionate about improving patient care. With a strong background in pre-clinical research&trial management and clinical monitoring, she has successfully contributed to Phase I-III studies during her time at the Erasmus Medical Centre, via Syneos (Contract Research Organization) and within the pharmaceutical company Bristol Myers Squibb. Furthermore, her expertise includes a bachelor’s in biomedical chemistry, and e.g. building onboarding programs and translating plans and ideas to concrete actions to ensure people have what they need to make the project a success.

Clinical Project & Management Assistant
Angelique brings a vast working experience, mostly in Human Resources of which 6 years as Director HR for Pharmaceutical Operations at MSD. With also an educational background in Business Administration, the broader picture is always on top of her mind. Life long learning as driving force and the passion for Sairopa’s field of expertise is what makes her tick in her daily work as Clinical Project & Management assistant.
To provide the best experiences, we use technologies such as cookies to store and/or access information on your device. By consenting to these technologies, we can process data such as browsing behavior or unique IDs on this site. If you do not give consent or withdraw your consent, this may adversely affect certain features and capabilities.